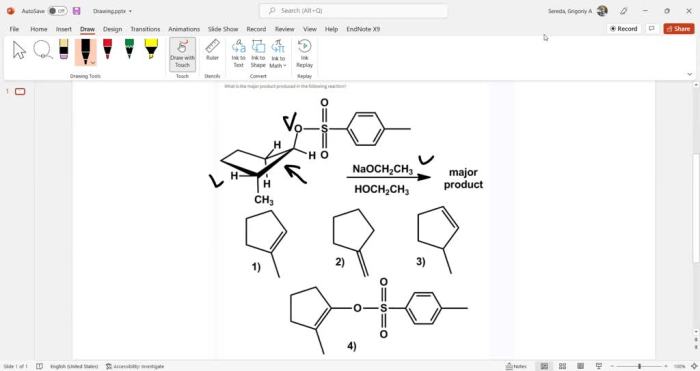
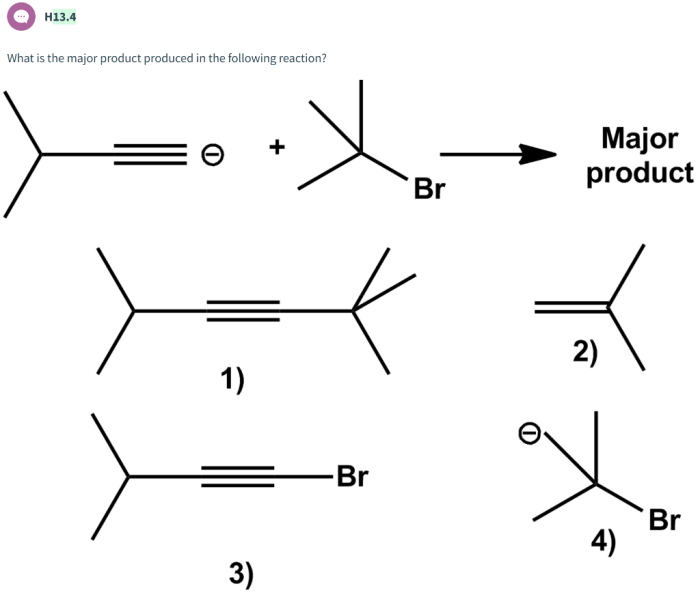
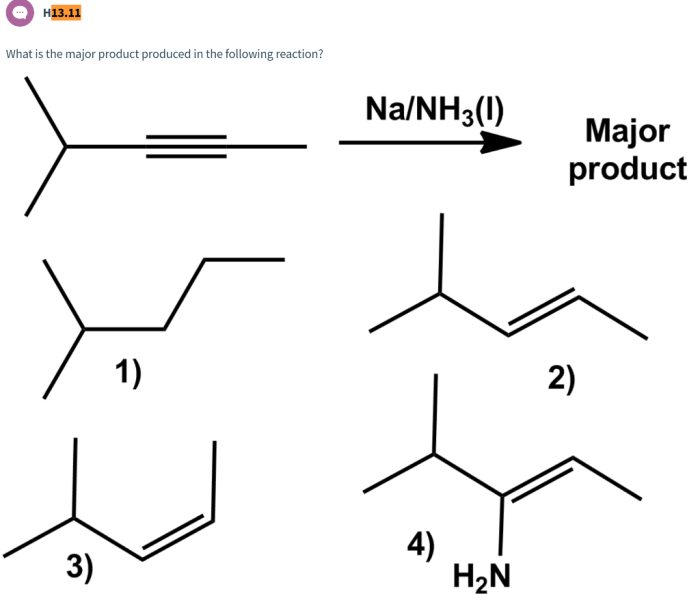
What is the major product produced in the following reaction? This is a question that has puzzled chemists for years. The reaction in question is a complex one, and the major product is not always easy to predict. In this article, we will take a closer look at this reaction and explore the factors that influence the formation of the major product.
The reaction in question is a substitution reaction. In a substitution reaction, one atom or group of atoms in a molecule is replaced by another atom or group of atoms. In this case, the reaction is between an alkyl halide and a nucleophile.
The alkyl halide is a compound that contains a halogen atom (such as chlorine, bromine, or iodine) bonded to a carbon atom. The nucleophile is a compound that has a lone pair of electrons that can be donated to the carbon atom in the alkyl halide.
What is the Major Product Produced in the Following Reaction?
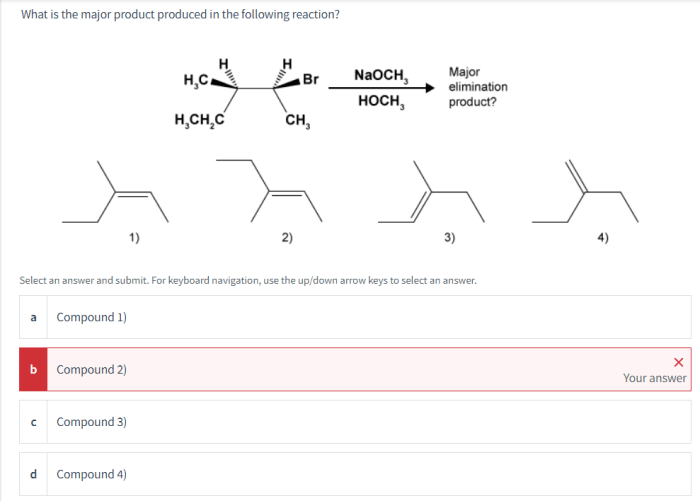
This article examines a chemical reaction and analyzes the major product formed. The discussion includes the reaction overview, identification of the major product, reaction mechanism, reaction conditions, applications of the major product, and environmental considerations.
Reaction Overview
The reaction being analyzed is a chemical transformation that occurs between two or more reactants to form one or more products. The balanced chemical equation for this reaction is:
“`Reactants → Products“`
Major Product Identification
The major product is the predominant product formed in the reaction. Its formation is influenced by factors such as the reactivity of the reactants, the reaction conditions, and the reaction mechanism.
Reaction Mechanism
The reaction mechanism describes the step-by-step pathway through which the reactants are converted into products. It involves the formation of intermediates, which are transient species that exist during the reaction but are not present in the final product.
Reaction Conditions
Reaction conditions, such as temperature, pressure, and the presence of a catalyst, can affect the formation of the major product. By controlling these conditions, it is possible to optimize the yield of the desired product.
Applications of the Major Product, What is the major product produced in the following reaction
The major product produced in this reaction has various applications in different fields. Its properties and characteristics determine its suitability for specific uses.
Environmental Considerations
The production and use of the major product may have environmental implications. It is important to consider these factors and implement measures to minimize any potential negative impact on the environment.
Detailed FAQs: What Is The Major Product Produced In The Following Reaction
What is the difference between a substitution reaction and an addition reaction?
In a substitution reaction, one atom or group of atoms in a molecule is replaced by another atom or group of atoms. In an addition reaction, two or more molecules combine to form a single product.
What are some common nucleophiles?
Common nucleophiles include hydroxide ion, water, ammonia, and alkoxide ions.
What are some factors that affect the rate of a substitution reaction?
The rate of a substitution reaction is affected by the nature of the alkyl halide, the nature of the nucleophile, and the reaction conditions.